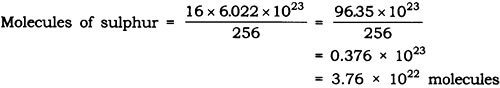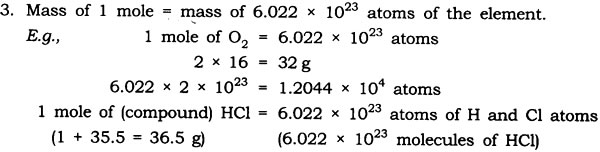Question: What is the mass of:
- 0.2 mole of oxygen atoms?
- 0.5 mole of water molecules?
Answer:
- Mole of Oxygen atoms = 0.2 mole
Molar mass of oxygen atoms = 16 g
Mass of oxygen atoms = 16 x 0.2 = 3.2 g - Mole of water molecule = 0.5 mole
Molar mass of water molecules = 2 x 1 + 16= 18 g .
Mass of H2O = 18 x 0.5 = 9 g
Answer:
The law of conservation of mass states that in a closed system, the mass of the system cannot change over time. Look at our example of the candle in the closed room. Though much of the wax itself is no longer present in its original form, all of the mass of the wax is still present in the room, albeit in a different form.
When the flame was lit, oxygen gas from the room reacted with the candle wax to produce water vapor and carbon dioxide gas. If you massed the reactants oxygen and wax, it would equal the mass of the products water and carbon dioxide. We can remember the law of conservation of mass with this simple statement:
The mass of the reactants must equal the mass of the products.
Sadly for fans of magic, anything that has mass, including matter and energy, cannot be created or destroyed. That means, mass cannot simply appear out of nowhere and equally it cannot disappear. Matter may change forms however, giving the illusion of nothing out of something or vice versa, but the mass of the matter is always the same before and after the change. If 22 grams of reactants go into a chemical reaction, then 22 grams of products must be produced.
Question: Calculate the number of molecules of sulphur (S8) present in 16 g of solid sulphur.
Answer: Molar mass of S8 sulphur = 256 g = 6.022 x 1023 molecule
Given mass of sulphur = 16 g
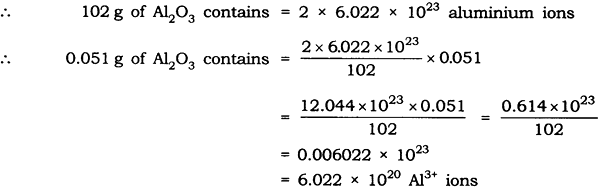
Question: Calculate the number of aluminium ions present in 0.051 g of aluminium oxide. (Hint: The mass of an ion is the same as that of an atom of the same element. Atomic mass of Al = 27 u)
Answer: Molar mass of aluminium oxide Al203
= (2 x 27) + (3 x 16)
= 54 + 48 = 102 g.
Question:
- How do atoms exist?
- What is atomicity?
- What are polyatomic ions?
Answer:
- Atoms of some elements are not able to exist independently. For such elements atoms form molecules and ions. In case of metals and inert gases atoms can exist independently.
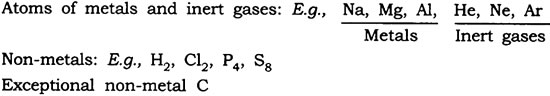
- The number of atoms constituting a molecule is known as its atomicity.
E.g.,O3 —> atomicity is 3
O2 —> atomicity is 2 - Polyatomic ions: When more than two atoms combine together and act like an atom with a charge on it is called polyatomic ion.
E.g., OH–, N03–, NH4+
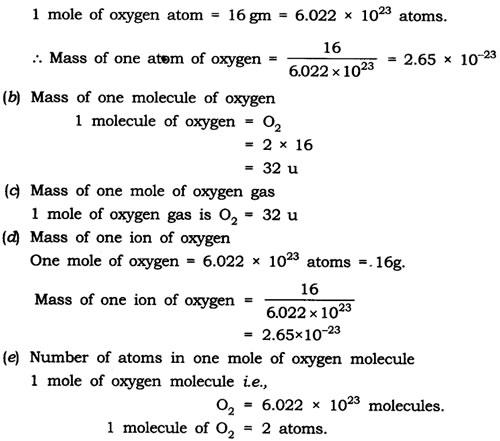
Question: Calculate
(a) the mass of one atom of oxygen
(b) the mass of one molecule of oxygen
(c) the mass of one mole of oxygen gas
(d) the mass of one ion of oxygen
(e) the number of atoms in 1 mole of oxygen molecule
Answer:
(a)
Question: What is meant by atomic mass, gram atomic mass of an element? Why is the mass have different expressions i.e., ‘u’ and ‘g’?
Answer: The atoms are very tiny and their individual mass cannot be calculated as it is negligible. Hence the mass of atoms is expressed in units with respect to a fixed standard. Initially hydrogen atom with mass 1 was taken as standard unit by Dalton. Later, it was replaced by oxygen atom (0=16). But due to the isotopes the masses were found in fractions instead of whole number. Hence, carbon (C=12) isotope was taken as standard unit and was universally accepted.
The atomic mass unit is equal to one twelfth (1/12) the mass of an atom of carbon-12, its unit is u.
Gramatomic mass: When the atomic mass of an element is expressed in grams, it is called the gramatomic mass of the element.
The mass of atoms, molecules is expressed in ‘u’ and the mass of moles i.e., molar mass is expressed in g.
Question: Define a mole. Give the significance of the mole.
Answer: Mole – One mole of any species (atoms, molecules, ions or particles) is that quantity or number having a mass equal to its atomic or molecular mass in grams.
1 mole = 6.022 x 1023 in number (atoms, molecules, ions or particles)
Significance of the mole
1. A mole gives the number of entities present i.e, 6.022 x 1023 particles of the substance.
2. Mass of 1 mole is expressed as M grams.
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students