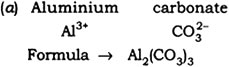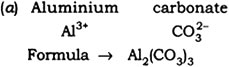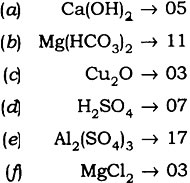Question: Calculate the formula unit mass of NaCl and CaCl2.
(Na = 23, Cl = 35.5, Ca = 40)
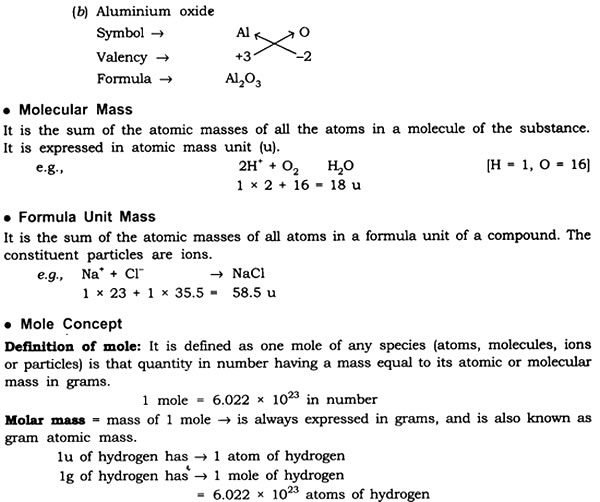
Answer: Formula unit mass of NaCl = 23 + 35.5
= 58.5 u
Formula unit mass of CaCl2 = 40 + (2 x 35.5)
= 40 + 71 = 111 u
Question: Write down the chemical formula for the following compounds:
(a) Aluminium carbonate
(b) Calcium sulphide
(c) Zinc carbonate
(d) Copper phosphate
(e) Magnesium bicarbonate
(f) Aluminium hydroxide.
Answer: The chemical formula are:
Question: Give the atomicity of the following compounds:
(a) Ca(OH)2 (d) H2S04
(b) Mg(HC03)2 (e) Al2(S04)3
(c) Cu20. (f) MgCl2
Answer: The atomicity of the molecules are:
Question: Explain the difference between 20, 02 and 03.
Answer: 2O —> It represents 2 atoms of oxygen (cannot exist independently).
O2 —> It represents one molecule of oxygen (made up of 2 atom) can exist freely.
O3 —> It represents one molecule of ozone (made up of 3 atoms) it can exist independently.
Question: Explain the difference between 2n and n2
Answer:
- 2N means two molecules of Nitrogen atom.
N2 means two atoms of nitrogen in its one molecules. - N2 shows that two nitrogen are bonded with covalent bond, and it become nitrogen gas whereas 2N does not show any bond, it just show two number of nitrogen.
- N2 is stable molecule, while 2N is nascent hydrogen with no stability.
Question: What is the difference between 2H and H2 ?
Answer: H2 is Molecular hydrogen. It is a molecule of hydrogen that consists of two hydrogen atoms bonded together by one single bond (also known as a sigma bond). A subscript also denotes how many of a particular atom is in a molecule, thus H2 has two hydrogens.
2H, on the other hand, denotes two moles of elemental hydrogen. It should be noted that elemental hydrogen is not bonded to anything.
Question: In a reaction 5.3 g of sodium carbonate reacted with 6 g of ethanoic acid. The products were 2.2 g of carbon dioxide, 0.9 g water and 8.2 g of sodium ethanoate. Show that these observations are in agreement with the law of conservation of mass carbonate.
Answer:
Question: Hydrogen and oxygen combine in the ratio of 1 : 8 by mass to form water. What mass of oxygen gas would be required to react completely with 3 g of hydrogen gas?
Answer: Ratio of H : O by mass in water is:
Hydrogen : Oxygen —> H2O
∴ 1 : 8 = 3 : x
x = 8 x 3
x = 24 g
∴ 24 g of oxygen gas would be required to react completely with 3 g of hydrogen gas.
Question: Which postulate of Dalton’s atomic theory is the result of the law of conservation of mass?
Answer: The postulate of Dalton’s atomic theory that is the result of the law of conservation of mass is—the relative number and kinds of atoms are constant in a given compound. Atoms cannot be created nor destroyed in a chemical reaction.
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students