Physical and Chemical Changes 7th Class NCERT CBSE Science Chapter 06
Question: What kind of change is shown by tearing of paper?
Answer: Tearing of paper is a physical change although, it cannot be reversed.
Question: Melting of wax is a change where a solid changes to liquid state. Give one more such change which you observe in your surroundings.
Answer: Melting of ice is also a change where solid changes into liquid state.
Question: Name the gas which turns lime water milky.
Answer: Carbon dioxide gas (CO2) turns lime water milky.
Question: Give example of a physical change which occurs by the action of heat.
Answer: Melting of ice to form water is a physical change which occurs by the action of heat.
Question: Write the colour of copper sulphate solution obtained when iron nails are dipped in it?
Answer: When iron nails are dipped in copper sulphate solution, then the colour of the solution changes to green.
Physical and Chemical Changes – Question: What colour of flame is observed when magnesium ribbon burnt in air.
Answer: When magnesium is burnt in air then a brilliant white flame is obtained.
Question: How can you say that ripening of a fruit is a chemical change?
Answer: Ripening of a fruit is a chemical change because after ripening, a new product with different properties is formed.
Question: Is souring of milk a physical change or a chemical change? Why?
Answer: Souring of milk is a chemical change because original substances present in milk lose their nature and identity and form new chemical substances.
Question: Complete the following reaction
Ca (OH)2 + CO2 →
Answer:
Ca (OH)2 + CO2 → CaCO3 + H2O
Question: What is the nature of magnesium oxide solution?
Answer: Magnesium oxide is basic in nature because it turns red litmus solution to blue.
Question: Name the process by which common salt is obtained from sea water.
Answer: The common salt can be obtained by the evaporation of sea water.
Physical and Chemical Changes – Question: Name the metal which is used for galvanizing iron.
Answer: Zinc metal is used for galvanizing iron.
Question: Name the metals which are mixed (alloyed) with iron to make stainless steel.
Answer: Metals like chromium and nickel are mixed (alloyed) with iron to make stainless steel.
Question: Suggest two methods to prevent rusting.
Answer: The two methods to prevent rusting are
- Painting the iron articles.
- Greasing or oiling the iron articles.
Question: We should eat freshly cut apple. Why?
Answer: We should eat freshly cut apple because if we leave the apple after cutting, it starts turn to brownish due to the oxidation of the essential nutrients present in it and its food value decreases.
Question: Write word equations for two chemical reactions with the help of materials given in the box.
Answer:
(i) Iron + air + water → iron oxide
(ii) Copper sulphate + iron → iron sulphate + copper
Questions Short Answer Type Questions
Question: Classify the following processes into physical or chemical changes.
- Beating of aluminium metal to make aluminium foil
- Digestion of food
- Cutting of a log of wood into pieces
- Burning of crackers
Answer: Physical changes are beating of aluminium metal to make aluminium foil and cutting of a log of wood into pieces. Chemical changes are digestion of food and burning of crackers.
Physical and Chemical Changes – Question: Explain the following:
- Lime water turns milky on passing carbon dioxide gas through it.Bubbles are produced when acetic acid is added to a solution of sodium hydrogen carbonate.
Answer:
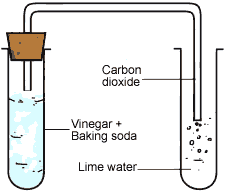
- Carbon dioxide gas produced in the reaction passing through freshly prepared lime water as shown in figure.
Set up to pass gas through lime water
Lime water is calcium hydroxide solution. When carbon dioxide gas is passed through lime water, then calcium hydroxide combines with carbon dioxide to form a white solid substance, calcium carbonate which makes lime water milky. This chemical change can be written in the form of word equation as follows:
Ca(OH)2 + CO2 → CaCO3 + H2O
The reaction between lime water and carbon dioxide gas is a chemical change because a new substance calcium carbonate is formed during this change. The turning of lime water into milky is a standard test of carbon dioxide.
(b) When baking soda (sodium hydrogen carbonate) and vinegar (acetic acid) are mixed together, then a chemical change takes place between sodium hydrogen carbonate and acetic acid to form three new substances.
The change in the test tube is as follows:
Sodium hydrogen carbonate + Acetic acid → Sodium acetate+ Carbon dioxide + Water
(Baking soda) (Vinegar)
Question: Is cloud formation a physical change or chemical change? Explain.
Answer: Formation of clouds is a physical change. Clouds are formed by the condensation of water vapours present in the atmosphere. When rainwater goes back on the earth, no new product is formed. Therefore, it is a physical change.
Question: Write the differences between physical and chemical changes.
Answer: Differences between physical and chemical changes are
Physical change:
- No new substance is formed.
- It is a temporary change.
- Physical change is easily reversible.
- Very little energy (heat, etc) is absorbed or given out in a physical change.
Chemical change:
- New substance is formed.
- It is a permanent change.
- Chemical change is irreversible.
- A lot of energy (in the form of heat, light, sound etc) is absorbed or given out in a chemical change.
Question: In addition to the formation of new products, what changes do the chemical changes accompany?
Answer: In addition to new products, the following may accompany a chemical change:
- Heat, light or any other radiation (e.g. ultraviolet) may be given off or absorbed.
- Sound may be produced.
- A change in smell may take place or a new smell may be given off.
- A colour change may take place.
- A gas may be formed.
Question: Magnesium ribbon bums in air and changes to white substance, i.e. magnesium oxide. When magnesium oxide dissolves in water, what type of change take place? Give reason in support of your answer. Express the change in the form of equation.
Answer: Mixing of ash obtained by the burning of magnesium with water is a chemical change. When magnesium is burnt in air, it forms magnesium oxide in the form of white ash.
Magnesium (Mg)+ Oxygen (O2) → Magnesium oxide (MgO)
When magnesium oxide dissolves in water, it forms a new substance, magnesium hydroxide.
Magnesium oxide (MgO) + Water (H2O) → Magnesium hydroxide Mg(OH)2
So, it is a chemical change.
Question: What is stainless steel? How is stainless steel made? State an important property of stainless steel.
Answer: Stainless steel is an alloy of iron. When iron is mixed (or alloyed) with carbon, chromium and nickel, then stainless steel is obtained. Stainless steel does not rust at all.
Question: Plants prepare their food by a process called photosynthesis. Can we call photosynthesis is a chemical change? Explain.
Answer: During photosynthesis, the plants intake carbon dioxide and water in the presence of chlorophyll and sunlight to form two new substances, i.e. glucose (food) and oxygen gas. So, photosynthesis is a chemical change.
Question: The process of digestion is a chemical change. Explain why.
Answer: In the process of digestion, the various food materials break down to form new substances which can be absorbed by the body. So, the process of digestion is a chemical change.
Question: How ozone layer acts as a protective shield?
Answer: The ozone layer protects us from the harmful ultraviolet radiations which come from the sun. Ozone absorbs ultraviolet radiations coming from the sun and breaks down to form oxygen.
In this way, ozone layer absorbs harmful ultraviolet radiations.
Question: Which type of change takes place in the following and state whether the energy is evolved or absorbed during the change?
Burning of a candle, lightning of a bulb, preparation of food by green plants, volcanic eruption, evaporation of petrol, burning of LPG.
Answer:
- Burning of a candle Chemical change as well as physical change and energy evolved.
- Lightning of a bulb Physical change, energy evolved.
- Preparation of food by green plants Chemical change, energy absorbed.
- Volcanic eruption Chemical change, energy evolved.
- Evaporation of petrol Physical change as no new chemical substance is formed, energy absorbed.
- Burning of LPG Chemical change because LPG on burning form CO2 and H2O,energy absorbed.
Question: Give two examples for each of the following cases:
- Physical changes which are reversible.
- Physical changes which are not reversible.
Answer:
- (a) Folding of paper
(b) Melting of ice - (a) Tearing of paper
(b) Breaking of glass
Question: Explosion of a cracker is a chemical change Explain.
Answer: When we burn a cracker, it explode Explosion produces heat, light, sound and unpleasant gases that pollute the atmosphere.
Many new products are formed. So, it is a chemical change.
Question: Why cannot a chemical change be normally reversed?
Answer: In a chemical change, the products are quite different from the reactants. Therefore, a chemical change cannot be normally reversed.
Question: A student took a solution of copper sulphate in a beaker and put a clean iron nail into it and left it for about an hour.
- What changes do you expect?
- Are these changes chemical in nature?
- Write a word equation for the chemical change, if any.
Answer:
- (a) Color of the solution in the beaker changes from blue to green.
(b) A brown colored deposit is found on the surface of the iron nail. - The changes are chemical in nature as new substances, iron sulphate (green) and copper (brown) are formed.
- Copper sulphate + Iron → Iron sulphate + Copper
(Blue) (Green) (Brown)
Question: Describe two changes that are harmful. Explain why you consider them harmful? How can you prevent them?
Answer: Harmful changes are
- Rusting of iron.
- Decaying of fruits.
Rusting of iron is harmful because it slowly destroys iron articles and makes them useless. Since, iron is used in making large number of objects or articles such as bridges, grills, railings, gates and bodies of cars, buses, trucks and ships, etc. Rusting of iron causes a great loss over a period of time.
Prevention Rusting can be prevented by oiling, greasing or painting. It can also be prevented by galvanization.
Decaying of fruits causes health hazards. Due to decaying of fruits, there is a lot of monetary loss in food industry.
Prevention Fruits can be preserved by keeping them at low temperature and by using some specific preservatives.
Question: What happens when an iron blade of a knife is dipped in a copper sulphate solution? What kind of change takes place?
Answer: When an iron blade of a knife is dipped in a copper sulphate solution, then iron blade is coated with reddish brown deposits of copper.
And the blue colour of copper sulphate solution changes to light green due to the formation of iron sulphate. So, it is a chemical change.
Physical and Chemical Changes: Long Answer Type Questions
Question: Give an example of a chemical reaction for each of the following situations:
- A change in colour is observed.
- A gas is evolved.
- Sound is produced.
Answer:
- Chemical reaction between copper sulphate solution and iron metal. In this reaction, blue colour of copper sulphate solution changes to light green colour due to the formation of iron sulphate.
Copper sulphate + Iron → Iron sulphate + Copper
(Blue) (Grey) (Light green) (Brown) - When baking soda and vinegar are mixed together then a chemical change takes place and bubbles of carbon dioxide gas are formed along with some other substances.
Baking soda + Vinegar → Sodium acetate + Carbon dioxide + Water
- Explosion of a firework produces heat, light, sound and unpleasant gases. Explosion of a firework is a chemical change.
Question: Rahul was a student of Class VII. His father purchased a new bicycle for him on his birthday. After few months, he found that the cycle chain and even the handle gets rusted. His father advised him to apply a coating of paint to the cycle and not to keep it in the open in future.
Now, answer the following questions:
- Why his cycle gets rusted?
- What do you mean by rusting of iron?
- What values are shown by Rahul’s father?
Answer:
- Rahul’s cycle was kept in the open for a longer time. As air contains both oxygen and moisture. Thus, in the presence of oxygen and water, his cycle slowly gets rusted.
Iron(Fe) + Oxygen + Water →Rust (Iron oxide) - If iron objects are left in humid conditions for a longer time, they get covered with reddish brown ferric oxide (Fe2O3) layer. This is called rusting of iron.
- Rahul’s father is caring, aware and intelligent.
Question: In the summer holidays, Karan went to Rann of Kutch in Gujarat with his parents. Karan was aware that in the coastal regions of India especially in the Rann of Kutch common salt is obtained from sea water. Karan was very excited to see that place. He requested his father that he want to see the process of obtaining salt from sea. His father helped him and they went to see the place where common salt was collecting.
And he also explained the whole process. Karan was very happy to see the process.
Now, answer the following questions.
- How is common salt obtained from sea water?
- Name the process by which salt is collected from sea water.
- What values are shown by Karan?
Answer:
- Sea water is collected in shallow pits. It is then allowed to evaporate in the sun. As, the water evaporates, the salt solution becomes supersaturated (concentrated). This supersaturated solution cannot hold the excess salt. Thus, it separates out in the form of salt crystals. These salt crystals are collected and are redissolved in water and filtered to remove insoluble impurities. The clear solution is again evaporated to obtain the crystals of pure salt.
- Salt is obtained from sea water by the process of evaporation.
- The values shown by Karan are curious, aware and intelligence.
Question: When baking soda is mixed with vinegar, bubbles are formed with the evolution of a gas. Name the gas evolved. What happens when this gas is passed through lime water?
Answer: When baking soda (sodium hydrogen carbonate) and vinegar (acetic acid) are mixed together, then a chemical change takes place between sodium hydrogen carbonate and acetic acid to form three new substances.
The change in the test tube is as follows:
Sodium hydrogen + Acetic acid → Sodium acetate + Carbon dioxide + water
Carbon dioxide gas produced in the reaction passing through freshly prepared lime water as shown in figure.
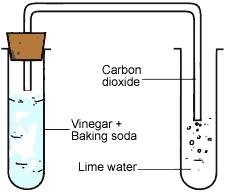
Set up to pass gas through lime water
Lime water is calcium hydroxide solution. When carbon dioxide gas is passed through lime water, then calcium hydroxide combines with carbon dioxide to form a white solid substance, calcium carbonate which makes lime water milky. This chemical change can be written in the form of word equation as follows:
Ca(OH)2 + CO2 → CaCO3 + H2O
The reaction between lime water and carbon dioxide gas is a chemical change because a new substance calcium carbonate is formed during this change. The turning of lime water into milky is a standard test of carbon dioxide.
When baking soda (NaHC03) reacts with vinegar which contains acitic acid carbon dioxide comes out, which turns lime water milky, therefore it is a chemical change. In all these activities, we saw that in each change, one or more new substances are formed. When magnesium ribbon was burnt, the ash was the new substance formed.
The reaction of copper sulphate with iron produced two new substances, i.e. iron sulphate and copper. Vinegar and baking soda together produced carbon dioxide which turned lime water milky. So, all those changes in which one or more new substances formed, are called chemical changes. These are permanent changes which can usually not be reversed to form the original substance.
Question: If you leave a piece of iron in the open for a few days, it acquires a film of brownish substance, called rust.
- Do you think rust is different from iron?
- Can you change rust back into iron by some simple method?
- Do you think formation of rust on iron is a chemical change?
- Give two other examples of a similar type of change.
Answer:
- Yes, rust is iron oxide (Fe2O3). Thus, rust and iron are not the same substance.
- No, rusting of iron is a chemical change because in this reaction, a new substance, rust (iron oxide) is formed. It cannot be reversed by any method.
- Yes, rusting of iron is a chemical change. During the rusting of iron, it combines with the oxygen in the presence of water (moisture) to form a new compound ‘iron oxide’. This iron oxide is a rust.
Iron + Oxygen + Water → Iron oxide
It is a permanent change which cannot be reversed back.
So, rusting of iron is a chemical change. - Two other examples are
(i) Setting of curd from milk.
(ii) Burning of magnesium ribbon to form magnesium oxide.
Fill in the Blanks
- Making sugar solution is a ………………… change.
- A physical change is generally ………………… .
- Grinding of wheat grain changes its size. It is a ………………… change.
- Iron benches kept in lawns and gardens get ………………… . It is a ………………… change because a new ………………… is formed.
- Some substances can be obtained in pure state from their solution by ……………….
- Energy is ………………… in the formation of curd from milk.
- The presence of ………………… in sea water makes the process of rust formation on ships faster.
- Souring of milk is a ………………… change.
- Melting of wax is a ………………… change but burning of wax is a change.
- The process of depositing a thin layer of zinc on iron object is called …………………
Answers:
- physical
- reversible
- physical
- rusted, chemical, substance
- crystallization
- evolved
- salt
- chemical
- physical, chemical
- galvanization
Physical and Chemical Changes: True / False
- When a candle bums, both physical and chemical changes take place.
- Anaerobic bacteria digest animal wastes and
- Ships suffer a lot of damage though they are painted.
- Stretching of rubber band is not a physical change.
- Cooking of rice is a physical change.
- Within our bodies, food is digested due to chemical reaction.
- A blue deposit is formed on an iron nail when dipped in copper sulphate solution.
- Both moisture and air are essential for rusting.
- Formation of clouds is a physical change.
- The colour of iron sulphate solution is green.
Answers:
- True
- True
- True
- False
- False
- True
- False
- True
- True
- True
Question: Match the items of Column I with the items of Column II.
| Column I | Column II |
| (a) Large crystals | (i) Turn lime water milky |
| (b) Depositing a layer of zinc on iron | (ii) Physical change |
| (c) Souring of milk | (iii) Rust |
| (d) Carbon dioxide | (iv) Sugar candy (mishri) |
| (e) Iron oxide | (v) Chemical change |
| (f) Dissolving common salt in water | (vi) Galvanization |
Answers:
- (a)-(iv)
- (b)-(vi)
- (c)-(v)
- (d)-(i)
- (e)-(iii)
- (f)-(ii)
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students