10 CBSE Board Science Paper 2018-19
Date: 13/03/2019
M.M.: 80
Class: 10th
Subject: Science
General Instructions:
- The question paper comprises five sections, A, B, C, D and E. You are to attempt all the sections.
- All questions are compulsory.
- Internal choice is given in sections B, C, D and E.
- Question numbers 1 and 2 in Section A are marks questions. They are to answered in one word or in one sentence.
- Question number 3 to 5 in Section B are two-marks questions. These are to be answered in about 30 words each.
- Question number 6 to 15 in Section C are three-marks questions. These are to be answered in about 50 words each.
- Question number 16 to 21 in Section D are five-marks questions. These are to be answered in about 30 words each.
- Question number 22 to 27 in Section E are based on practical skills. Each question is a two marks question. These are to be answered in brief.
Section A
Question: 1. If you could use any source of energy for heating your food which one would you prefer? State one reason for your choice. [1]
Question: 2. Write the function of voltmeter in an electric circuit. [1]
Section B
Question: 3. What happens to the image distance in the normal human eye when we decrease the distance of an object, say 10 m to 1 m ? Justify your answer. [2]
Question: 4. List two different functions performed by pancreas in our body. [2]
Question: 5. How it can be proved that the basic structure of the Modern Periodic Table is based on the electronic configuration of atoms of different elements? [2]
OR
The electronic configuration of an element is 2, 8, 4. State its:
(a) group and period in the Modern Periodic Table.
(b) name and write its one physical property.
Section C
Question: 6. How can we help in reducing the problem of waste disposal? Suggest any three methods. [3]
OR
Define an ecosystem. Draw a block diagram to show the flow of energy in an ecosystem.
Question: 7. List three advantages each of:
(i) exploiting resources with short term aims, and
(ii) using a long term perspective in managing our natural resources. [3]
Question: 8. What is a rainbow? Draw a labelled diagram to show the formation of a rainbow. [3]
Question: 9. Nervous and hormonal systems together perform the function of control and coordination in human beings. Justify this statement with the help of an example. [3]
Question: 10. Trace the sequence of events which occur when a bright light is focused on your eyes. [3]
Question: 11. What is photosynthesis? Explain its mechanism. [3]
Question: 12. Name the plant Mendel used for his experiment. What type of progeny was obtained by Mendel in F1 and F2 generations when he crossed the tall and short plants? Write the ratio he obtained in F2 generation plants. [3]
OR
List two differences between acquired traits and inherited traits by giving an example of each.
Question: 13. 2 g of silver chloride is taken in a china dish and the dish is placed in sunlight for sometime. What will be your observation in this case? Write the chemical reaction involved in the form of a balanced chemical equation. Identify the type of chemical reaction. [3]
OR
Identify the type of reactions taking in each of the following cases and write the balanced chemical equation for the reactions.
(a)Zinc reacts with silver nitrate to produce zinc nitrate and silver.
(b) Potassium iodide reacts with lead nitrate to produce potassium nitrate and lead iodide.
Question: 14. Based on the group valency of elements write the molecular formula of the following compounds giving justification for each:
(i) Oxide of first group elements.
(ii) Halide of the elements of group thirteen, and
(iii) Compound formed when an element, A of group 2 combines with an element, B of group seventeen. [3]
Question: 15. Explain the following:
(a) Sodium chloride is an ionic compound which does not conduct electricity in solid state where as it does conduct electricity in molten state as well as in aqueous solution.
(b) Reactivity of aluminium decrease if it dipped in nitric acid.
(c) Metals like calcium and magnesium are never found in their free state in nature. [3]
Section D
Question: 16.
(a) With the help of a suitable circuit diagram prove that the reciprocal of the equivalent resistance of a group of resistances joined in parallel is equal to the sum of the reciprocals of the individual resistances.
(b) In an electric circuit two resistors of 12 Ω each are joined in parallel to a 6 V battery. Find the current drawn from the battery.
OR
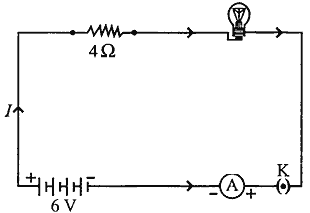
An electric lamp of resistance 20 Ω and a conductor of resistance 4 Ω are connected to a 6 V battery as shown in the circuit. Calculate:
(a) the total resistance of the circuit,
(b) the current through the circuit,
(c) the potential difference across the (i) electric lamp and (ii) conductor, and
(d) power of the lamp.
Question: 17.
(a) Draw magnetic field lines produced around a current carrying straight conductor passing through a cardboard. Name, state and apply the rule to mark the direction of these field lines.
(b) How will the strength of the magnetic field change when the point where magnetic field is to be determined is moved away from the straight wire carrying constant current? Justify your answer. [5]
Question: 18. An object is placed at a distance of 60 cm from a concave lens of focal length 30 cm.
(i) Use lens formula to find the distance of the image from the lens.
(ii) List four characteristic of the image (nature, position, size, erect / inverted) formed by the lens in this case.
(iii) Draw ray diagram to justify your answer of part (ii). [5]
Question: 19. Define pollination. Explain the different types of pollination. List two agents of pollination? How does suitable pollination lead to fertilization? [5]
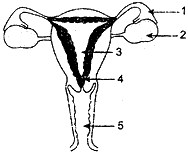
OR
(a) Identify the given diagram. Name the parts 1 to 5.
 Class Notes NCERT Solutions for CBSE Students
Class Notes NCERT Solutions for CBSE Students